Add your promotional text...
The Phosphorus Paradox: Why Plants Starve on a Full Plate, and How Microbes Can Help
Source & Further Information: The findings and concepts discussed in this article are largely based on the research presented in the following scientific paper: Ducousso-Détrez A, Fontaine J, Lounès-Hadj Sahraoui A, Hijri M. Diversity of Phosphate Chemical Forms in Soils and Their Contributions on Soil Microbial Community Structure Changes. Microorganisms. 2022 Mar 13;10(3):609. doi: 10.3390/microorganisms10030609. PMID: 35336184; PMCID: PMC8950675. We encourage readers interested in the detailed methodology and complete results to consult the original publication.
8/25/20254 min read
The Phosphorus Problem: A Locked-Up Feast
Phosphorus (P) is absolutely essential for life. It's a core building block for DNA, cell membranes, and the energy currency (ATP) that powers every living plant. Farmers know this well – for decades, high-yield agriculture has relied on adding phosphate fertilizers to ensure crops have enough of this vital macronutrient.
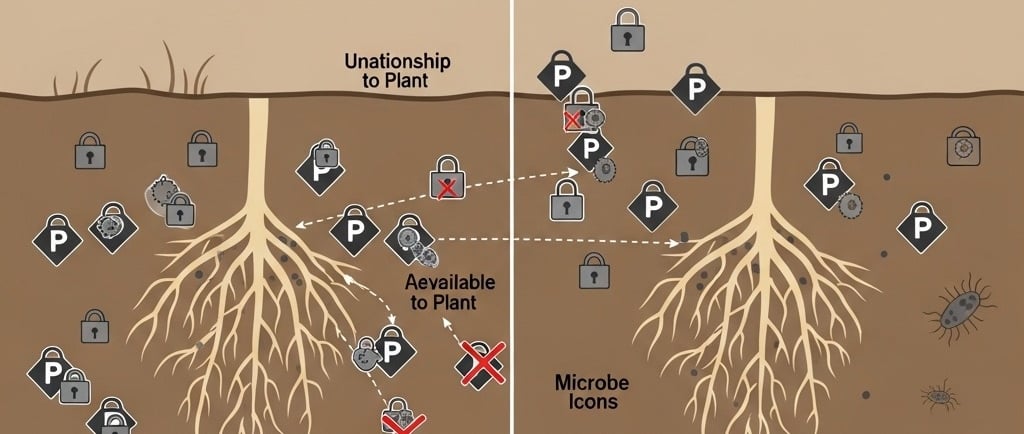
However, this has created a paradox. To boost yields, we've often overused P fertilizers, which is costly and can lead to environmental damage like water pollution (eutrophication). But the bigger issue is that a huge portion of the phosphorus we add—up to 80% shortly after application—doesn't even stay available to plants! It gets rapidly "locked up" by the soil itself, forming insoluble mineral complexes that plants simply can't absorb. It's like serving a feast in a locked safe; the food is there, but no one can get to it.
This leaves us with a critical challenge: how can we manage this locked-up phosphorus legacy and make it available to plants in a more sustainable way? The answer may lie with some of the smallest residents of the soil: microbes.
Meet the Microbial Miners: Phosphorus-Solubilizing Microbes (PSM)
Living within the complex world of the soil are billions of microorganisms. This "soil microbiota" acts as a massive, dynamic reservoir for nutrients, including phosphorus. Within this community are special groups known as Phosphate-Solubilizing Microbes (PSM). These bacteria and fungi are nature's expert miners. They have the remarkable ability to break down insoluble, locked-up phosphorus compounds and convert them into a soluble form (orthophosphate ions) that plant roots can readily slurp up.
The potential of these tiny helpers is enormous. Researchers have been exploring the idea of using PSM as "bio-fertilizers" or "inoculants" for years. The goal is to enrich the soil with these beneficial microbes to improve natural phosphorus cycling and reduce our reliance on chemical fertilizers. Some studies even suggest that effective PSM inoculation could cut phosphate fertilizer needs by as much as 50%!
However, the reality in the field can be complicated. The performance of these microbial inoculants can be inconsistent, and scientists are still working to understand the intricate dance between soil chemistry, the native microbial community, and the specific phosphorus forms present. To develop truly effective bio-fertilizers, we need to look deeper into this three-way relationship.
The Many Faces of Phosphorus: Not All P is Created Equal
A key piece of the puzzle is understanding that "phosphorus" in the soil isn't just one thing. It exists in a huge diversity of chemical forms, broadly split into two pools:
Inorganic Phosphorus: This is P bound to minerals. The primary source is apatite, the mineral that makes up 95% of the Earth's crustal phosphorus. In acidic soils, P tends to bind with iron and aluminum. In alkaline soils, it's often locked up with calcium. These forms can be very stable and unavailable to plants.
Organic Phosphorus: This is P incorporated into organic molecules, mostly from decomposed plants, animals, and microbes. It can make up 30-65% of total P in many soils. Key forms include phosphate esters (like inositol phosphates from plants) and diesters (like DNA and RNA from dead cells).
Only a tiny fraction—much less than 1%—of the total phosphorus in soil is ever dissolved in the soil water at one time, ready for uptake. The rest is locked in this complex web of inorganic and organic forms. The constant "dance" between being locked up (sorption/precipitation) and becoming available (desorption/dissolution) is what controls P availability.
Does Phosphorus Shape the Microbial Community?
If different microbes specialize in accessing different forms of P, then it stands to reason that the amount and type of phosphorus in the soil should influence which microbes thrive there. Researchers have studied this extensively, and the results are both fascinating and complex.
Countless studies have confirmed that adding phosphorus fertilizer does change the soil microbial community. But the results are often contradictory. For example:
Some studies find that adding P boosts the abundance of beneficial Arbuscular Mycorrhizal Fungi (AMF), while others find it suppresses them. It seems to depend on the specific AMF species and the type of P added.
Similarly, certain bacterial groups, like Proteobacteria and Firmicutes (which contain many known PSM), show different responses depending on the fertilizer type (e.g., rock phosphate vs. superphosphate) and soil conditions.
In some experiments, high P levels favored fast-growing bacteria, while low-P conditions favored slower-growing, more specialized microbes.
Confusingly, one study might find a higher bacterial diversity in P-rich soil, while another finds higher diversity in P-poor soil.
The Missing Link: It's Not Just About How Much P, but What Kind
So why the conflicting results? This review highlights a critical gap in much of the research: studies often measure the impact of P by looking at just "total P" or "available P." They rarely account for the full diversity of chemical P forms present and how those forms change after fertilizer is applied.
The type of fertilizer used (rock phosphate, manure, various chemical blends) introduces a completely different starting set of P compounds. What happens next depends entirely on the soil's properties (its pH, clay content, organic matter) and climate (temperature, moisture), which control how that P is transformed and locked up.
The authors propose a crucial hypothesis: the dynamics and diversity of P forms in the soil likely have a more significant effect on microbial communities than just the simple addition of phosphorus fertilizer.
A Path Forward: Connecting Chemistry and Microbiology
To create the next generation of effective bio-fertilizers, we need to stop looking at soil phosphorus and soil microbes in isolation. The future of this research lies in a multidisciplinary approach:
Deeper P Analysis: We need to pair studies of microbial communities with advanced analysis of the specific chemical forms of phosphorus present in the soil.
Understanding Interactions: We must better understand the complex, multi-kingdom interactions between bacteria, fungi, and plants. Network analysis can help reveal which microbes tend to work together.
Dynamic Modeling: Ultimately, the goal is to build models that can predict how P will transform in a given soil, allowing us to design microbial inoculants that are truly effective for specific conditions.
By connecting the dots between soil chemistry and microbiology, we can move closer to unlocking the vast reserves of phosphorus already present in our soils, fostering a more sustainable and resilient agricultural future.